Meet Michael Spencer
Tell me a little about yourself!
I am a 4th year PhD student in Materials Science and Engineering, working in Dr. Veronica Augustyn’s research group. I am also the current vice president of the SciBridge club that supplies experiment kits related to sustainable energy technologies to universities in East Africa! After graduation, I would like to continue studying materials for electrochemical energy storage and catalysis and pursue a career that allows me to inspire and equip future generations of scientists with the practical knowledge they need to achieve their goals. For fun, I spend as much time outside as possible. Recently, I’ve enjoyed hiking, fishing, and playing disc golf.

At AIF, I use the FEI Verios scanning electron microscope, the Rigaku Smartlab X-ray diffractometer, and the FEI Talos transmission electron microscope to understand the structure of the materials that are synthesized in our lab. I like to use these instruments because they provide fundamental insights into the materials at dimensions far smaller than what we can perceive, and it can be directly correlated with, in my case, the electrochemical performance.
What have you been researching and how is it impacting the community?
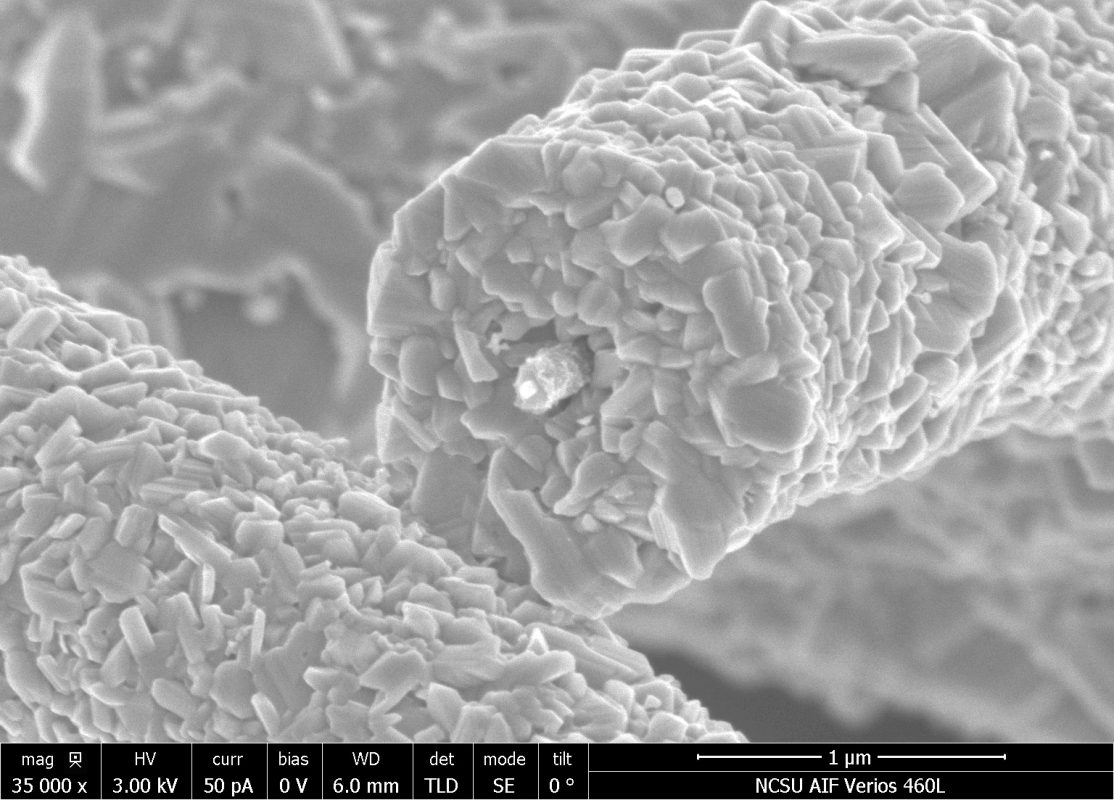
The majority of my research thus far has focused on the development of a free-standing 3D electrode architecture for Li-ion batteries. The composite electrodes consist of an aligned carbon nanotube (CNT) foam and molybdenum trioxide, which is conformally coated on the surface of the CNTs via electrodeposition. The electrode design provides good electron and ion transport that enables high charge storage at reasonably high rates for battery-type materials. My current work is focused on understanding how electrochemical reactivity of small molecules and redox active species changes when they are confined within nanometer and sub-nanometer spaces rather than in bulk solutions.
We hope to design materials that can improve the efficiency of electrochemical reactions such as the hydrogen evolution reaction. This work could influence a huge market for hydrogen production that is used for metal refining, for heating, and as fuel. Current hydrogen production techniques predominantly involve the burning of hydrocarbons, which is costly and simultaneously produces carbon dioxide.
Successfully developing materials that make electrochemical hydrogen production more efficient would be more cost effective and drastically reduce carbon emissions for this industry.
What have you learned from your experience at AIF?
I continuously learn about the intricate details that lead to acquiring good quality data, and whether it is my own work or the work of others, I am always impressed with the capabilities of the equipment at AIF.
Is there a staff member at AIF that has helped you?
I would specifically like to recognize Chuck and BB for all that they have taught me about the instruments and for their continuous assistance as new questions arise with each session!
- Categories: